Introduction:
Outcomes of patients with relapsed or refractory multiple myeloma (MM) have improved substantially with access to novel therapeutic agents. However, patients with triple class exposed (TCE) MM continue to have poor outcomes. Though newer bispecific antibody and chimeric antigen receptor T-cell immunotherapies have improved outcomes of TCE patients, these therapies are inaccessible outside of clinical trials in many publicly funded healthcare systems, including Canada. This study aimed to describe treatment patterns, outcomes, unplanned health care utilization and quality-of-life impairments of TCE MM patients in Ontario, Canada. These data will provide a crucial benchmark to compare to as novel immunotherapies become available.
Methods:
This retrospective observational study utilized data from the Institute for Clinical Evaluative Sciences (IC/ES) administrative database, which contains all health records of patients treated within Ontario's publicly funded healthcare system. Drug exposure was determined using the Cancer Activity Level Reporting (ALR) and Ontario Drug Benefit Claims Registry (ODB) databases, housed within IC/ES, which contain data on intravenous and oral MM treatment exposure for standard of care and clinical trial regimens. Patients were defined as TCE if they had prior or current treatment with a regimen containing an immunomodulatory drug (lenalidomide or pomalidomide), a proteasome inhibitor (bortezomib, carfilzomib, or ixazomib), and an anti-CD38 monoclonal antibody (isatuximab or daratumumab). The TCE index regimen was identified as the treatment on which a patient was first identified as having met the TCE definition. Inpatient hospitalization visits did not include planned chemotherapy infusion visits. Quality of life was described using the Edmonton Symptom Assessment System Score with scores ³ 7 corresponding to severe symptoms. Overall survival (OS) was determined using the Kaplan-Meier method.
Results:
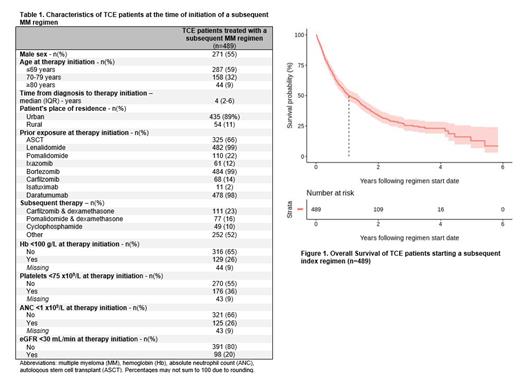
Of the 16,777 patients with a diagnosis of MM between 2007-2021 in Ontario, 1358 patients were classified as TCE during their treatment course. The TCE index regimen was a daratumumab-based regimen in most patients [daratumumab/velcade/dex (n=566, 42%), daratumumab/lenalidomide/dex (n=619, 46%)]. Of the TCE patients, 247 (18%) were still receiving treatment with the TCE index regimen at last follow up, 263 (19%) died without receiving any additional treatment, and 489 (36%) received a subsequent MM treatment after the TCE index regimen.
Baseline characteristics of patients (n=489) treated with a subsequent MM regimen after post TCE are summarized in Table 1. The three most commonly administered therapies post TCE were carfilzomib-dexamethasone (n=111, 23%), pomalidomide-dexamethasone (n=77, 16%) and oral cyclophosphamide (n=49, 10%). The next-line treatment regimen was administered in an academic versus community treatment center in 235 (48%) versus 254 (52%) of patients, respectively. The next-line therapy was a clinical trial in only 37 (8%) patients. Regarding healthcare utilization, among TCE patients receiving subsequent therapy, 149 (30%) were evaluated in the emergency department (ED), 126 (26%) were admitted to hospital, and 48 (10%) were referred to palliative care while on treatment with the subsequent therapy. Among patients with available information, the most common severe patient-reported symptoms during the subsequent line of treatment included: tiredness (27%), pain (23%) and overall poor well-being (21%). Additionally, 35% of patients had a ECOG PS of ≥2 on next-line treatment. The median OS patients treated after the TCE index regimen was 1.05 year (95% CI 0.89-1.35) as shown in Figure 1. Of the patients that died during follow up, the majority died in hospital (n=147, 44%) and were referred to palliative care (217, 65%).
Conclusion:
In this real-world study, we showed that the overall outcomes of TCE MM patients remains poor in Canada, highlighting the urgent need for novel therapies. Furthermore, we demonstrated that TCE patients receiving subsequent therapy had significant unplanned health care utilization and poor quality of life. Future studies will need to assess whether the outcomes, healthcare utilization, and patient-reported quality of life improve as newer immunotherapies become accessible as standard of care treatments within our publicly funded healthcare system.
Disclosures
Visram:Janssen: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Apotex: Consultancy, Honoraria. Pond:Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Profound Medical: Consultancy; Astra-Zeneca: Consultancy; Merck: Consultancy. McCurdy:Janssen: Honoraria; GSK: Honoraria; Celgene: Honoraria; Amgen: Honoraria; Takeda: Honoraria; Sanofi: Honoraria; Forus therapeutics: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria. Sapru:Forus therapeutics: Consultancy, Honoraria; Apotex: Consultancy, Honoraria; Janssen: Consultancy, Honoraria. Aljama:Pfizer, Sanofi and Beigene: Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Foley:Janssen: Speakers Bureau; Novartis: Speakers Bureau; Gilead: Speakers Bureau. Mian:Amgen: Honoraria; Janssen: Honoraria, Research Funding; Celgene / BMS: Honoraria; Takeda: Honoraria; Sanofi: Honoraria; GSK Awards: HHS Research Early Career Award from Hamilton Health Sciences Foundation: Honoraria; Roche: Current equity holder in publicly-traded company; Forus: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal